RxNorm
RxNorm, стандартизированная номенклатура для клинических лекарственных средств, произведенная для National Library of Medicine. В этом контексте клинический препарат является фармацевтическим продуктом, данным (или взятым) пациентом с терапевтическим или диагностическим намерением. В RxNorm название клинического препарата объединяет свои компоненты, преимущества и формы.
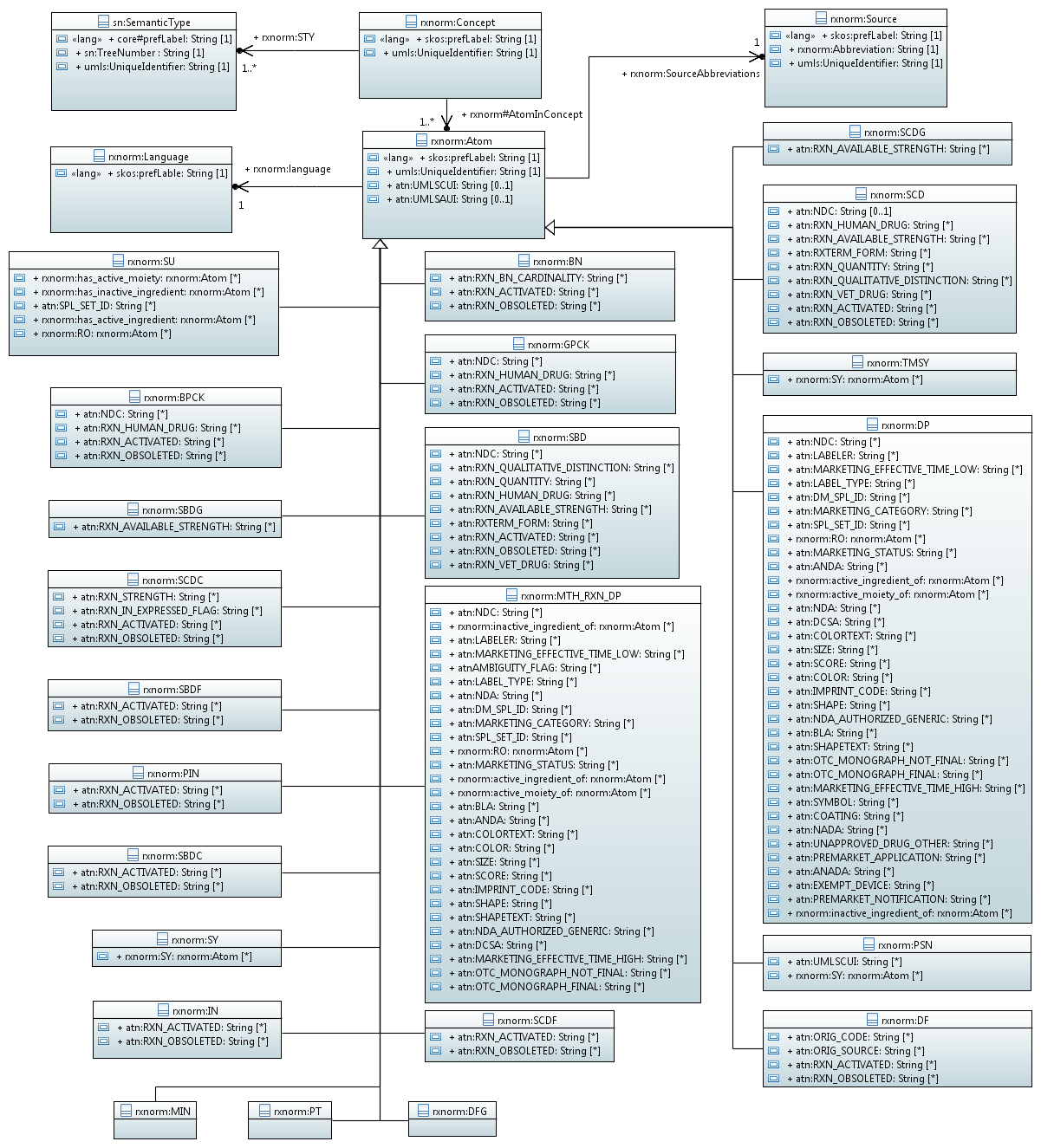
Базовые классы RxNorm
- rxnorm:Concept - Основные понятия
- rxnorm:Atom - Основные понятия
- sn:SemanticType - Классификационные понятия семантической сети UMLS
- rxnorm:Language - Язык представления данных
- rxnorm:Souкce - Источники данных
Классы
rxnorm:BN - Fully-specified drug brand name that can not be prescribed
- atn:RXN_BN_CARDINALITY - Cardinality of RxNorm Brand Name Atom
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:BPCK - Branded Drug Delivery Device
- atn:NDC - National Drug Code corresponding to a clinical drug (e.g. 000023082503)
- atn:RXN_HUMAN_DRUG - Drug available for use in Humans
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:DF - Dose Form
- atn:ORIG_CODE - Original code associated with this string
- atn:ORIG_SOURCE - Original source associated with this string
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:DFG - Dose Form Group
rxnorm:DP - Drug Product
- atn:NDC - National Drug Code corresponding to a clinical drug (e.g. 000023082503)
- rxnorm:inactive_ingredient_of - Inactive Ingredient Of
- atn:LABELER - FDA Structured Product Label Manufacturer/Distributor/Supplier name
- atn:MARKETING_EFFECTIVE_TIME_LOW - The date the MTHSPL drug became completed
- atn:LABEL_TYPE - DailyMed label type
- atn:DM_SPL_ID - DailyMed internal identifier for MTHSPL atom
- atn:MARKETING_CATEGORY - Marketing category
- atn:SPL_SET_ID - FDA Structured Product Label SET_ID code
- rxnorm:RO - Has relationship other than synonymous, narrower, or broader
- atn:MARKETING_STATUS - The Marketing Status of the MTHSPL drug
- atn:ANDA - Abbreviated New (Generic) Drug application number for the MTHSPL drug
- rxnorm:active_ingredient_of - Active ingredient of
- rxnorm:active_moiety_of - Active Moiety of
- atn:NDA - New Drug Application number for MTHSPL drug
- atn:DCSA - Controlled Substance Act designation code (e.g. 0,2,3n)
- atn:COLORTEXT - FDA Structured Product Label imprint attribute for color text
- atn:SIZE - FDA Structured Product Label imprint attribute for size
- atn:SCORE - FDA Structured Product Label imprint attribute for score
- atn:COLOR - FDA Structured Product Label imprint attribute for color
- atn:IMPRINT_CODE - Imprint Code
- atn:SHAPE - FDA Structured Product Label imprint attribute for shape
- atn:NDA_AUTHORIZED_GENERIC - New Drug Application number for authorized generic MTHSPL drug
- atn:BLA - Therapeutic Biologic Applications number for the MTHSPL drug
- atn:SHAPETEXT - FDA Structured Product Label imprint attribute for shape text
- atn:OTC_MONOGRAPH_NOT_FINAL - FDA Structured Product Label OTC monograph status
- atn:OTC_MONOGRAPH_FINAL - FDA Structured Product Label OTC monograph status
- atn:MARKETING_EFFECTIVE_TIME_HIGH - The date the MTHSPL drug became active
- atn:SYMBOL - FDA Structured Product Label imprint attribute for symbol
- atn:COATING - FDA Structured Product Label imprint attribute for coating
- atn:NADA - New Animal Drug Application number for MTHSPL drug
- atn:UNAPPROVED_DRUG_OTHER - Marketing category unapproved_drug_other for MTHSPL atom
- atn:PREMARKET_APPLICATION - Premarket Application
- atn:ANADA - Abbreviated New Animal Drug application number for the generic drug for MTHSPL
- atn:EXEMPT_DEVICE - Exempt Device
- atn:PREMARKET_NOTIFICATION - FDA Structured Product Label premarket notification
rxnorm:GPCK - Generic Drug Delivery Device
- atn:NDC - National Drug Code corresponding to a clinical drug (e.g. 000023082503)
- atn:RXN_HUMAN_DRUG - Drug available for use in Humans
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:IN - Name for an ingredient
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:MIN - Name for a multi-ingredient
rxnorm:MTH_RXN_DP - RxNorm Created DP
- atn:NDC - National Drug Code corresponding to a clinical drug (e.g. 000023082503)
- rxnorm:inactive_ingredient_of - Inactive Ingredient Of
- atn:LABELER - FDA Structured Product Label Manufacturer/Distributor/Supplier name
- atn:MARKETING_EFFECTIVE_TIME_LOW - The date the MTHSPL drug became completed
- atn:AMBIGUITY_FLAG - Source atom ambiguity flag
- atn:LABEL_TYPE - DailyMed label type
- atn:NDA - New Drug Application number for MTHSPL drug
- atn:DM_SPL_ID - DailyMed internal identifier for MTHSPL atom
- atn:MARKETING_CATEGORY - Marketing category
- atn:SPL_SET_ID - FDA Structured Product Label SET_ID code
- rxnorm:RO - Has relationship other than synonymous, narrower, or broader
- atn:MARKETING_STATUS - The Marketing Status of the MTHSPL drug
- rxnorm:active_ingredient_of - Active ingredient of
- rxnorm:active_moiety_of - Active Moiety of
- atn:BLA - Therapeutic Biologic Applications number for the MTHSPL drug
- atn:ANDA - Abbreviated New (Generic) Drug application number for the MTHSPL drug
- atn:COLORTEXT - FDA Structured Product Label imprint attribute for color text
- atn:COLOR - FDA Structured Product Label imprint attribute for color
- atn:SIZE - FDA Structured Product Label imprint attribute for size
- atn:SCORE - FDA Structured Product Label imprint attribute for score
- atn:IMPRINT_CODE - Imprint Code
- atn:SHAPE - FDA Structured Product Label imprint attribute for shape
- atn:SHAPETEXT - FDA Structured Product Label imprint attribute for shape text
- atn:NDA_AUTHORIZED_GENERIC - New Drug Application number for authorized generic MTHSPL drug
- atn:DCSA - Controlled Substance Act designation code (e.g. 0,2,3n)
- atn:MARKETING_EFFECTIVE_TIME_HIGH - The date the MTHSPL drug became active
- atn:OTC_MONOGRAPH_NOT_FINAL - FDA Structured Product Label OTC monograph status
- atn:OTC_MONOGRAPH_FINAL - FDA Structured Product Label OTC monograph status
rxnorm:PIN - Name from a precise ingredient
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:PSN - Prescribable Names
- rxnorm:SY - Synonym
- atn:UMLSAUI - UMLS atom unique identifier
rxnorm:PT - Designated preferred name
rxnorm:SBD - Semantic branded drug
- atn:NDC - National Drug Code corresponding to a clinical drug (e.g. 000023082503)
- atn:RXN_QUALITATIVE_DISTINCTION - RXN Qualitative Distinction
- atn:RXN_QUANTITY - Normal Form quantity factor
- atn:RXN_HUMAN_DRUG - Drug available for use in Humans
- atn:RXN_AVAILABLE_STRENGTH - Available drug strengths listed in the order of ingredients from the drug
- atn:RXTERM_FORM - The RxTerm dose form name for this drug
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
- atn:RXN_VET_DRUG - Drug available for use in animals
rxnorm:SBDC - Semantic Branded Drug Component
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:SBDF - Semantic branded drug and form
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:SBDG - Semantic branded drug group
- atn:RXN_AVAILABLE_STRENGTH - Available drug strengths listed in the order of ingredients from the drug
rxnorm:SCD - Semantic Clinical Drug
- atn:NDC - National Drug Code corresponding to a clinical drug (e.g. 000023082503)
- atn:RXN_HUMAN_DRUG - Drug available for use in Humans
- atn:RXN_AVAILABLE_STRENGTH - Available drug strengths listed in the order of ingredients from the drug
- atn:RXTERM_FORM - The RxTerm dose form name for this drug
- atn:RXN_QUANTITY - Normal Form quantity factor
- atn:RXN_QUALITATIVE_DISTINCTION - RXN Qualitative Distinction
- atn:RXN_VET_DRUG - Drug available for use in animals
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:SCDC - Semantic Drug Component
- atn:RXN_STRENGTH - Strength plus unit of SCDC
- atn:RXN_IN_EXPRESSED_FLAG - Strength Expressed As Precise Flag
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:SCDF - Semantic clinical drug and form
- atn:RXN_ACTIVATED - Date the RxNorm atom was reactivated
- atn:RXN_OBSOLETED - Date the RxNorm atom became obsolete
rxnorm:SCDG - Semantic clinical drug group
- ATN:RXN_AVAILABLE_STRENGTH - Available drug strengths listed in the order of ingredients from the drug
rxnorm:SU - Active Substance
- rxnorm:has_active_moiety - Has Active Moiety
- rxnorm:has_inactive_ingredient - Has Inactive Ingredient
- atn:SPL_SET_ID - FDA Structured Product Label SET_ID code
- rxnorm:has_active_ingredient - Has active ingredient
- rxnorm:RO - Has relationship other than synonymous, narrower, or broader
rxnorm:SY - Source asserted synonymy
- rxnorm:SY - Source asserted synonymy.
rxnorm:TMSY - Tall Man synonym
- rxnorm:SY - Source asserted synonymy